strontium electron configuration|Iba pa : Bacolod Mar 23, 2023
88 Plaza Hotel, Iloilo City: See 20 traveller reviews, 7 user photos and best deals for 88 Plaza Hotel, ranked #209 of 372 Iloilo City B&Bs / inns and rated 3 of 5 at Tripadvisor. . Prices are provided by our partners, and reflect average nightly room rates, including taxes and fees that are fixed, known to our partners, and due at time of .
PH0 · strontium electron configuration full
PH1 · full electron configuration of platinum
PH2 · electron configuration for every element
PH3 · electron configuration for dummies
PH4 · electron configuration chart pdf
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · electron config strontium
PH8 · Iba pa
Subscribe for moreee. If you like it, give it a thumbs up.Flexing my hot kababayan.#flex_ph_official #trending #viral #flex #pinay #stunningfilipina
strontium electron configuration*******The ground state electron configuration of strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. This electron configuration shows that the last shell of strontium has two electrons. Therefore, the valence electrons of strontiumare two. The elements that have 1, 2, or 3 electrons in the last shell . Tingnan ang higit pa
The total number of electrons in strontium is thirty-eight. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in strontium in specific rules in different . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa
Wayne Breslyn. 736K subscribers. 433. 63K views 4 years ago. In order to write the Sr electron configuration we first need to know the number of electrons for . Mar 23, 2023 Strontium is a chemical element of the periodic table with chemical symbol Sr and atomic number 38 with an atomic weight of 87.621 u and is classed as alkaline earth metal and .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
Electron configuration for Sr is termed as the distribution of electrons in its orbits. Strontium consists of 38 electrons. Electron configuration of Strontium is given as below: 1s 2 2s 2 2p 6 3s 2 3p 6 . Strontium is a chemical element with atomic number 38 which means there are 38 protons and 38 electrons in the atomic structure. The chemical symbol for .Strontium. Full electron configuration of strontium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. rubidium ← strontium → yttrium. Strontium, complete electron configuration. The strontium electron configuration, denoted as 5s 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2, illustrates the arrangement of electrons within the atom. This configuration can be determined . Key Takeaways. The electron configuration of strontium is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^6 6s^2. Strontium has 38 electrons . Strontium is a chemical element with the symbol Sr and atomic number 38. It belongs to the alkaline earth metal group on the periodic table. The electron configuration of an atom describes how its electrons are distributed among the various atomic orbitals.In the case of strontium, the electron configuration can be . Strontium is an alkaline earth metal. It is soft and has Silvery white and yellowish metallic element. Strontium is a highly reactive element. When Strontium is exposed to air it makes a dark oxide layer. .Strontium. Full electron configuration of strontium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. rubidium ← strontium → yttrium. Strontium, complete electron configuration.Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells.Iba pa To write the configuration for the Strontium ions, first we need to write the electron configuration for just Strontium (Sr). We first need to find the numb.The electron configuration of strontium is as 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2.Strontium atom loses two valence electrons from its s-orbital to attain noble gas configuration. What is the complete electron configuration of strontium? Electron shell configuration: [Kr]5s^2; Melting Point (K): 1042; Boiling Point(K): 1657; . Strontium-90, a radioactive isotope of the metal produced by fission reactions is a dangerous environmental menace because its chemistry is similar to calcium and it may take its place in bones. The strong radiation emitted by the isotope interferes .Strontium is a chemical element of the periodic table with chemical symbol Sr and atomic number 38 with an atomic weight of 87.621 u and is classed as a alkaline earth metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 5s 2: Electrons per shell: 2, 8, 18, 8, 2: Valence electrons : 2: Valency electrons : 2:The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Strontium is [Kr] 5s2. Possible oxidation states are +2.Electron configuration 5s 2: Electrons per shell: 2, 8, 18, 8, 2: Physical properties; . Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. La configuration électronique d'un atome décrit comment ses électrons sont répartis dans différents niveaux d'énergie ou orbitales. Dans le cas du strontium (Sr), qui possède un numéro atomique de 38, le configuration électronique en c'est 2+ ion (Sr2+) est légèrement différent de l'atome neutre.
icon : plus-circle. Protons et neutrons dans le Strontium. Le strontium est un élément chimique de numéro atomique 38, ce qui signifie qu’il y a 38 protons dans son noyau. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole Z.. La charge électrique totale du noyau est donc +Ze, où e (charge .
Electron Configuration. Strontium has an electron configuration of [Kr] 5s². It has 38 electrons orbiting its nucleus, with the outermost electrons residing in the 5s subshell. This electron configuration is particularly significant because it dictates strontium's propensity to lose two electrons, forming ions with a +2 charge. .
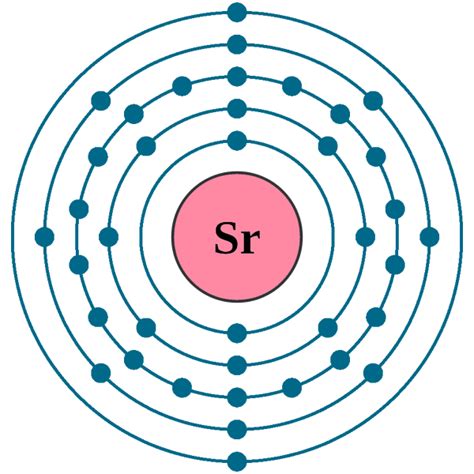
La configuration électronique complète du strontium est 1s2 – 2s2 – 2p6 – 3s2 – 3p6 – 3d 10 -4s2 – 4p6 – 5s2. Le strontium fait partie du tableau périodique. C'est un élément chimique dont le numéro atomique est 38, son symbole est M. Il se caractérise par être un métal mou, sa couleur est brillante et argentée, il a une .strontium electron configurationLa configuration électronique complète du strontium est 1s2 – 2s2 – 2p6 – 3s2 – 3p6 – 3d 10 -4s2 – 4p6 – 5s2. Le strontium fait partie du tableau périodique. C'est un élément chimique dont le numéro atomique est 38, son symbole est M. Il se caractérise par être un métal mou, sa couleur est brillante et argentée, il a une .The full Electron configuration of strontium is 1s2 – 2s2 – 2p6 – 3s2 – 3p6 – 3d 10 -4s2 – 4p6 – 5s2. Strontium is part of the periodic table. It is a chemical element whose atomic number is 38, its symbol is M.Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some historic information. Periodic Table of the Elements; Strontium: . Electronic data Shells: 2, 8, 18, 8, . Strontium-90 is a long lived highly radioactive fallout product of atomic-bomb explosions.

Now the atomic number of strontium (Sr) is 38. Hence the electron arrangement in strontium is 2, 8, 18, 8, 2. And the electron configuration of strontium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2. This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of strontium atom .
strontium electron configuration Iba pa Now the atomic number of strontium (Sr) is 38. Hence the electron arrangement in strontium is 2, 8, 18, 8, 2. And the electron configuration of strontium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2. This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of strontium atom .
That is, you could write Na: 1s 2 2s 2 2p 6 3s 1 or [Ne]3s 1 noting that [Ne]=1s 2 2s 2 2p 6. Figure 7.2.3 7.2. 3: For sodium it is better to use the convention of expressing the core electrons with that of a Nobel gas. Figure 7.2.4 7.2. .
How to say Cambodia Phnom Penh in English? Pronunciation of Cambodia Phnom Penh with 1 audio pronunciation and more for Cambodia Phnom Penh.
strontium electron configuration|Iba pa